Drug Safety Evaluation
A guide for authors submitting to the Expert Collection
Scope
Drug Safety Evaluations focus on providing an independent perspective on the safety of a specific drug. The purpose of the Drug Safety Evaluation is to promote best practice in use of the drug and should therefore be limited to approved indications and avoid off-label discussion.
Word limit
The word limit for Drug Safety Evaluations is 3,000 words.
Every article must contain
Titles should be concise but informative, and contain no brand names. They should also be impartial and non-promotional.
Including address, academic qualifications and job titles of all authors, as well as telephone number, fax number and email address of the author for correspondence on a separate cover sheet as the peer reviewers will not be aware of the authors’ identity.
Please note that only the address of the first author of the article will appear on Medline/PubMed, not necessarily the corresponding author.
Please note that only the address of the first author of the article will appear on Medline/PubMed, not necessarily the corresponding author.
Maximum 200 words.
The aim of the abstract is to draw in the interested reader and provide an accurate reflection of the content of the paper. We therefore request the following structure is followed for full-length review articles:
Introduction: Authors are required to describe the significance of the topic under discussion.
Areas covered: Authors are required to describe the research discussed and the literature search undertaken.
Expert Opinion: Authors are required to summarize briefly their Expert Opinion section.
References must not be included in the abstract.
The aim of the abstract is to draw in the interested reader and provide an accurate reflection of the content of the paper. We therefore request the following structure is followed for full-length review articles:
Introduction: Authors are required to describe the significance of the topic under discussion.
Areas covered: Authors are required to describe the research discussed and the literature search undertaken.
Expert Opinion: Authors are required to summarize briefly their Expert Opinion section.
References must not be included in the abstract.
A brief list of keywords, in alphabetical order, is required to assist indexers in cross-referencing. The keywords will encompass the therapeutic area, mechanism(s) of action, key compounds etc.
Incorporating basic information on disease incidence and prevalence, unmet medical need and present treatment guidelines (highlighting regional variations where appropriate).
Introduction:
Background to the development and use of the drug
Body of review:
– Mechanism of action, including key PK/PD data
– Clinical applications, including key efficacy data
Safety evaluation (note, this section should form the main part of the review):
– Safety in clinical studies
– Postmarketing data
– Safety in special populations, including pharmacogenomic data if available
Comparison with safety of other drugs:
Include a table comparing data if appropriate
Conclusion:
An analysis of the data presented in the review
Background to the development and use of the drug
Body of review:
– Mechanism of action, including key PK/PD data
– Clinical applications, including key efficacy data
Safety evaluation (note, this section should form the main part of the review):
– Safety in clinical studies
– Postmarketing data
– Safety in special populations, including pharmacogenomic data if available
Comparison with safety of other drugs:
Include a table comparing data if appropriate
Conclusion:
An analysis of the data presented in the review
To distinguish reviews published in the series of journals, authors must provide an additional section entitled Expert Opinion. This section affords authors the opportunity to go beyond the conclusion and provide their interpretation of the data presented in the article.
Authors should answer the following:
1. What, if any, improvement does the drug hold over other therapies?
2. What, if any, impact is this drug likely to have on current treatment strategies?
3. How likely are physicians to prescribe the drug?
4. What data is still needed?
5. Where is the drug likely to be in five years’ time?
Please note that ‘opinions’ are encouraged in the Expert Opinion section, and as such, referees are asked to keep this in mind when peer reviewing the manuscript.
Authors should answer the following:
1. What, if any, improvement does the drug hold over other therapies?
2. What, if any, impact is this drug likely to have on current treatment strategies?
3. How likely are physicians to prescribe the drug?
4. What data is still needed?
5. Where is the drug likely to be in five years’ time?
Please note that ‘opinions’ are encouraged in the Expert Opinion section, and as such, referees are asked to keep this in mind when peer reviewing the manuscript.
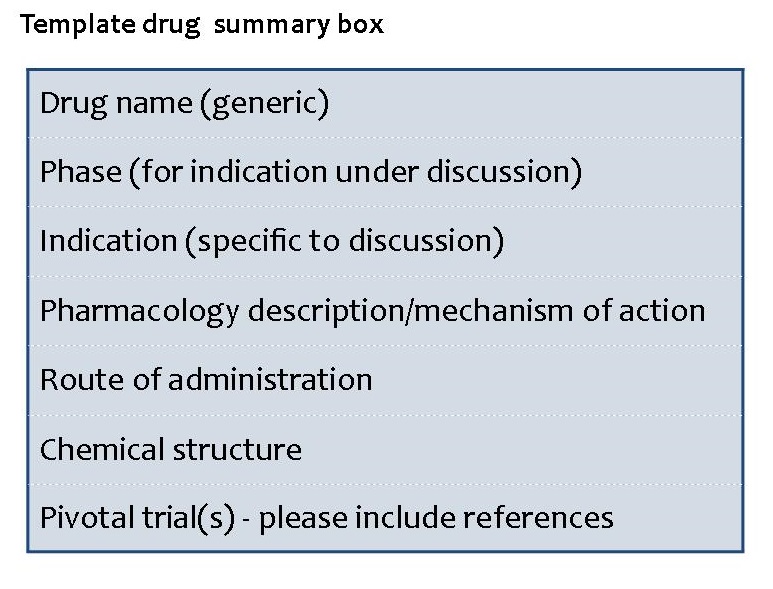
To provide the reader with a visual summary of the paper, each paper should include a drug summary box including basic data on the drug as follows:

A maximum of 100 references is suggested. Ensure that all key work relevant to the topic under discussion is cited in the text and listed in the bibliography. Reference to unpublished data should be kept to a minimum and authors must obtain a signed letter of permission from cited persons to use unpublished results or personal communications in the manuscript.
Important references should be highlighted with a one/two star system and brief annotations should be given.
Up to five figures and five tables are permitted. For further information on tables and figures, please see our formatting guide.
Explore the Expert Collection
Discover all Expert Collection, journal specific guidelines.