Drug Evaluation: Expert Opinion on Biological Therapy
A guide for authors submitting to the Expert Collection
Scope
Drug Evaluations will present an evaluated overview of the clinical experience with the biologic compound. Although discussion should encompass basic pharmacology and pharmacokinetics, the primary focus of the review should be the clinical efficacy of the compound. For launched compounds the information should be limited to approved indications and avoid off-label discussion.
Peer review
All articles are subject to peer review by 2-3 independent reviewers. As the Drug Evaluations include in-depth analysis of a single compound, the manuscript will also be sent to the company responsible for marketing or developing the drug to check the accuracy of the data. This is for a technical check only and the final editorial decision is based entirely on the recommendation of the independent peer review. Comments remain confidential and are shared only with the corresponding author or submitting party.
Explore the Expert Collection
Discover all Expert Collection, journal specific guidelines.
Word limit
The word limit for Drug Evaluations is 3,000 words (not including tables, figures and references).
Every article must contain
Introduction: Authors are required to describe the significance of the topic under discussion.
Areas covered: Authors are required to describe the research discussed and the literature search undertaken.
Expert opinion: Authors are required to summarize briefly their Expert opinion section.
References must not be included in the abstract.
– What are the unmet needs of currently available therapies?
– Which competitor compounds/classes of compounds are in the clinic/late development?
Introduction to the compound
Chemistry; Pharmacodynamics; Pharmacokinetics and metabolism.
Clinical efficacy
(Phase I studies); Phase II studies; Phase III studies; Post-marketing surveillance.
Safety and tolerability
Regulatory affairs
Conclusion
An analysis of the data presented in the review.
1. What, if any, improvement does the drug hold over other therapies?
2. What, if any, impact is this drug likely to have on current treatment strategies?
3. How likely are physicians to prescribe the drug?
4. What data is still needed?
5. Where is the drug likely to be in five years’ time?
Please note that ‘opinions’ are encouraged in the Expert opinion section, and as such, referees are asked to keep this in mind when peer reviewing the manuscript. However, authors are requested to focus their discussion on approved uses of the drug.
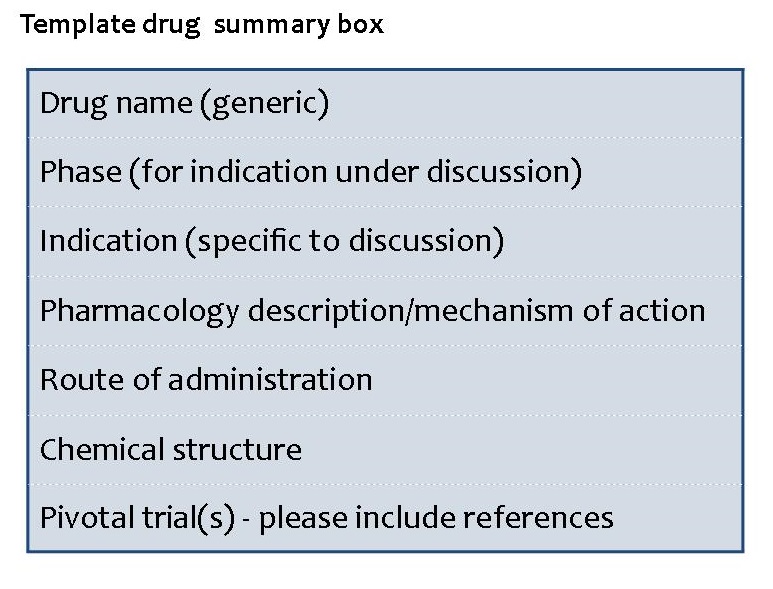
If you are unable to provide this information, editorial support will be provided.