Registered Reports at Taylor & Francis
Explore journals offering Registered Reports
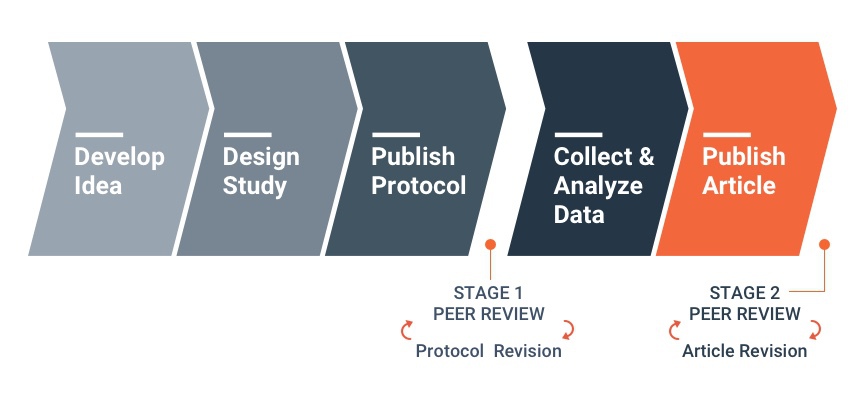
What is a Registered Report?
The Center for Open Science describes Registered Reports as “a publishing format…that emphasizes the importance of the research question and the quality of methodology by conducting peer review prior to data collection”. In practice this means changing the way research is conducted and published.
Background and benefits
A Registered Report is a form of empirical article offered by the journal. The methods and proposed analyses are submitted to the journal for review prior to research being conducted. High-quality protocols are then provisionally accepted for publication and are pre-registered before data collection commences.
This format is designed to improve research design while minimizing publication bias other issues in hypothesis-driven research. It allows for the flexibility to conduct exploratory (unregistered) analyses and report unplanned findings.
Registered Reports aim to reduce questionable research practices such as P-hacking, Cherry Picking, HARKing.
Publishing tips, direct to your inbox
Expert tips and guidance on getting published and maximizing the impact of your research. Register now for weekly insights direct to your inbox.
Help with your Registered Report submission
Interested in submitting a registered report but don’t know where to start? We’ve got you covered. Get detailed information on submitting a Registered Report.